A crystal – but not as we know it
10 March 2023
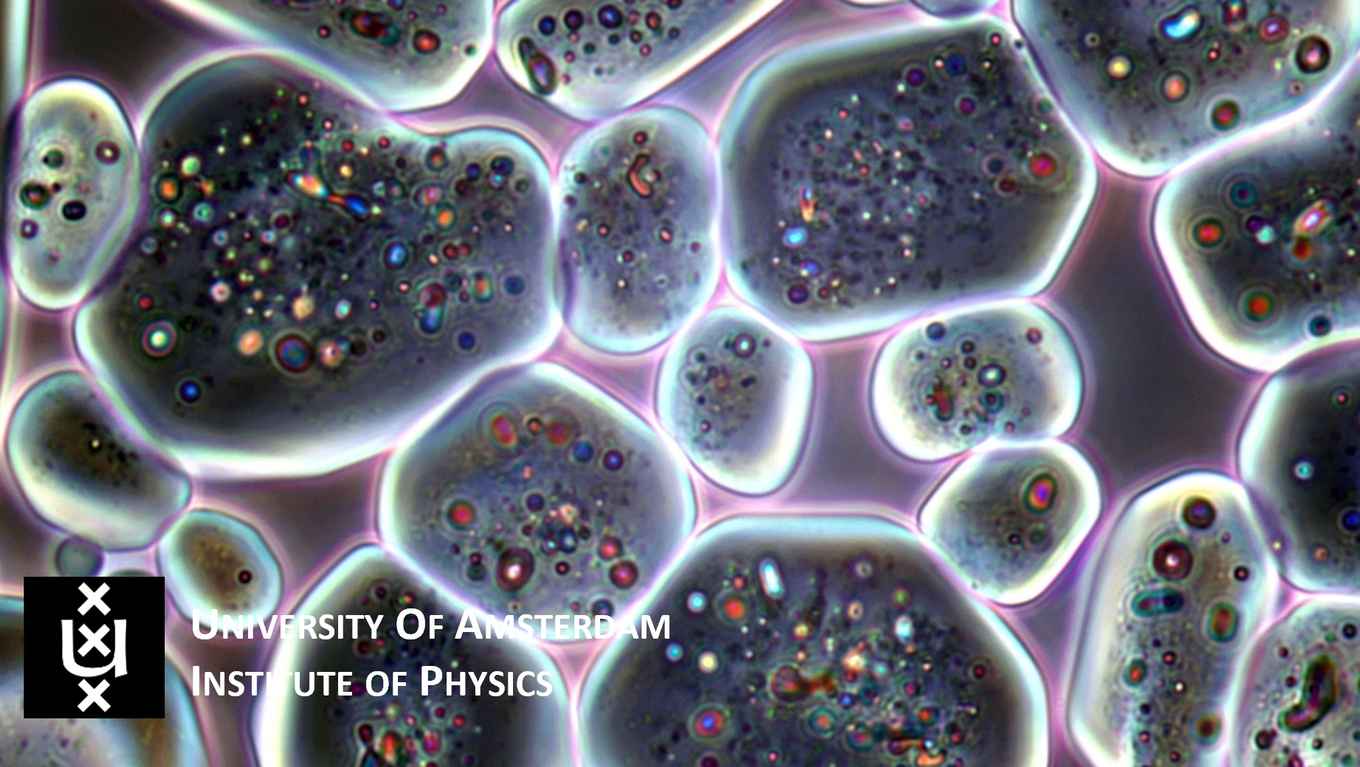
Floppy crystals
Crystals are generically hard solids, and are usually identified by their well-defined geometrical shape that reflects the underlying highly ordered molecular structure. In their paper, the physicists show that surprisingly, some salts that contain water in their crystalline structure (so-called hydrated salts) can behave remarkably differently. When these salts are slowly dissolved through contact with humid air, they become soft, deformable and lose their facets. This is in contrast to regular crystals, that keep their faceted shape and stay hard while dissolving. Thus, the microcrystals that were studied simultaneously are crystalline in the bulk of the material, but show liquid-like molecular mobility at their surfaces.
Editors’ Highlight
The paper was published in Nature Communications and was selected by the editors of that journal as one of the featured articles for the Editors’ Highlights webpage of recent research in the section Materials science and chemistry. The Editors’ Highlights pages aim to showcase the 50 best papers recently published in an area.
Publication
Softness of hydrated salt crystals under deliquescence, Rozeline Wijnhorst, Menno Demmenie, Etienne Jambon-Puillet, Freek Ariese, Daniel Bonn and Noushine Shahidzadeh. Nature Communications 14 (2023) 1090.